Stem Cell Imaging
Stem cell transplants provide unprecedented opportunities for reconstitution of previously incurable tissue injuries. However, therapeutic cells migrate, proliferate, differentiate, and respond to their environment. We are interested in leveraging our expertise in nanomedicine and stem cell biology towards the development of clinical imaging tools for in vivo monitoring of these processes. Our team developed patented, clinically translatable technologies for direct differentiation of induced pluripotent stem cells into mesenchymal stromal cells (US14/210,752). We applied this method for the development of personalized drug sensitivity assays. We also introduced immediately clinically applicable imaging technologies for in vivo tracking of stem cell transplants: Currently, success of stem cell transplants is diagnosed several months after their implantation by evaluation of the degree of tissue repair. Our imaging technologies accelerate diagnoses by direct depiction of stem cell engraftment (Nanomedicine 2012), stem cell apoptosis (ACS Nano 2014) or immune rejection (Radiology 2012 and 2017). We currently apply our in vivo stem cell imaging technique to track stem cell engraftment in osteonecroses in pediatric leukemia patients. These imaging tools provide a truly new way to evaluate stem cell physiology beyond simple cell detection, expanding our understanding of in vivo engraftment outcomes.
Stem Cell Imaging
Monitoring of Stem Cell Engraftment in Arthritic Joints
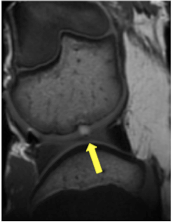
Cartilage defects are the major source of pain in arthritic joints. Current treatments, whilst alleviating some of the clinical symptoms, prove insufficient to cure the underlying irreversible cartilage loss. Stem cells represent a unique source for restoration of cartilage defects. However, a major challenge with various approaches of stem cell and chondrocyte transplants is lack of engraftment, death of the transplanted cells and resultant insufficient defect repair. Matrix associated stem cell implants (MASI) represent a promising approach for treatment of arthritis. We are developing imaging tests for in vivo tracking of stem cell engraftment and for non-invasive diagnoses of complications of the engraftment process, such as MASI dislocation from the transplant site, stem cell death due to apoptosis or immune responses that lead to rejection. Studies in animal models are ongoing and studies in patients will follow soon.
Imaging Chondrogenic Differentiation of human iPS Cells
In order to regenerate hyaline cartilage in arthritic joints, transplanted stem cells must not only survive and engraft, but also differentiate into chondrocytes. We are developing novel, "smart" imaging approaches for non-invasive in vivo visualization of in vivo differentiation processes of induced pluripotent stem cells (iPS cells) into chondrocytes, using activatable contrast agents and multi-modality imaging technologies.
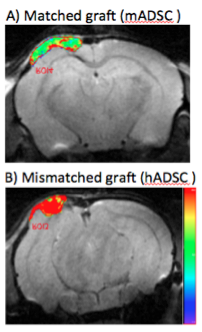
Imaging Host Immune Responses to Stem Cell Transplants
Bone injuries are amongst the most costly and debilitating to individuals and our society. Achieving successful repair of large bone defects represents one of the most challenging problems in reconstructive surgery. Stem cell-scaffold nanocomposites have many advantages over bone grafts, such as higher tissue regeneration potential, immediate availability, potentially unlimited quantities and generally better engraftment outcomes. However, allogeneic adult stem cells, embryonic stem cell-derived progenitors and even induced pluripotent stem cells can possess major and minor histocompatibility antigen differences, which can be recognized as foreign by the host immune system and lead to their rejection. Considering that the use of allogeneic ("off the shelf") stem cells is commonly advocated, and thus rejection may be a common occurrence, a non-invasive diagnostic test for in vivo detection of stem cell rejection is urgently needed to detect transplant failure early enough for corrective interventions. We are developing novel imaging tests for in vivo tracking of macrophages and T-cells into stem cell transplants, which could lead to rejection. Information from these new imaging tests may facilitate timely recognition and treatment of host immune responses to stem cell transplants, and ultimately, better engraftment outcomes.

